2 Marks Question
- What happens when carbon dioxide is passed through lime water for a long time? Write with equation.
-
If the carbon dioxide is continued to pass for a long time, it turns into
colorless due to formation of solution sodium bicarbonate.
CaCO3 + H2O + CO2 ⟶ Ca(HCO3) (colorless soluble) - Which chemical is used to obtain dry ammonia? Why?
- Quick Lime (CaO) is used to obtain dry ammonia because it removes moisture and does not react with ammonia to form unwanted products.
-
Method of preparing of which gas is shown in figure? Write the balanced
equation of chemical reaction of preparing that gas.
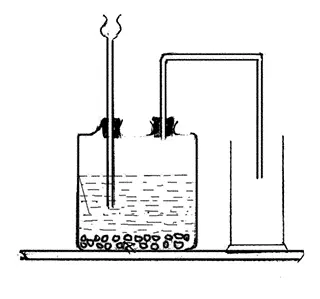
-
The method of preparation of carbon dioxide gas is shown in the figure.
It is prepared in lab by the reaction between dilute hydrochloric acid (HCl), marble pieces (CaCO3).
CaCO3 + HCl ⟶ CaCl2 + H2O + CO2 ↑ - Why is carbon dioxide gas collected by upward displacement of air?
- Carbon dioxide gas is heavier than air. So, it is collected by upward displacement of air.
- Which gas is collected in lab by downward displacement of air? Why?
- Ammonia gas is collected in the lab by downward displacement of air because it is lighter than air and soluble in water.
- What happen when carbon dioxide gas is passed into lime water for short period? Write with balanced chemical equation.
-
When carbon dioxide gas is passed into lime water for short period milky
color appear due to formation of insoluble calcium carbonate
(CaCO3).
Ca(OH)2 + CO2 ⟶ CaCO3 ↓ + H2O -
Study the adjoining figure and answer the following questions.
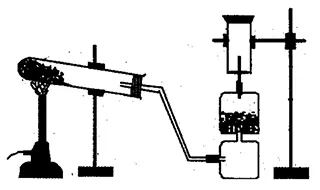
-
- Which gas can be prepared by the given apparatus?
- Which gas can be prepared by the given apparatus?
- Lime tower is used in this process, why?
- During the reaction between ammonium chloride and calcium hydroxide, water is formed along with ammonia. Hence, Calcium oxide present in the lime tower helps to dry the ammonia gas.
- Write balanced chemical equation of the chemical reaction which takes place between two chemicals, generally kept in the fire extinguisher.
-
The balance chemical equation of the chemical reaction which takes place
between two chemicals, generally kept in the fire extinguisher (Carbon
dioxide) is:
CaCO3 + HCl ⟶ CaCl2 + H2O + CO2 ↑
CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2 ↑ - How is carbon dioxide manufactured in the industry? Write with chemical equation.
-
Carbon dioxide manufactured in the industry by heating calcium carbonate in
big furnace.
CaCO3 CaO + CO2 - Which gas is produced by the chemical reaction between dilute hydrochloric acid and marble pieces? Write the balanced chemical equation.
-
Carbon dioxide gas is produced by the chemical reaction between dilute
hydrochloric acid and marble pieces.
CaCO3 + HCl ⟶ CaCl2 + H2O + CO2 ↑
CaCO3 + 2HCl ⟶ CaCl2 + H2O + CO2 ↑ - Write any two necessary conditions for the industrial production of ammonia? The mixture of carbon dioxide gas and water is sour, why?
-
Necessary conditions for the industrial production of ammonia are:
- Temperature should be maintained between 400-500°C.
- Nitrogen and hydrogen should be in 1:3 ratio.
Most of the acids are sour in taste. Carbonic acid is formed by the mixture of carbon dioxide gas and water. So, the mixture of carbon dioxide gas and water is sour.
CO2 + H2O ⟶ H2CO3 (Carbonic acid) - What happens if ammonia gas is passed into water containing few drops of phenolphthalein, why?
- If ammonia gas is passed into water containing a few drops of phenolphthalein, it turns pink because ammonia is basic gas.
- Lime water turns to milky when carbon dioxide gas is passed through it, why?
-
Lime water turn to milky when carbon dioxide gas is passed through it
because due to formation of insoluble calcium carbonate (CaCO3).
Ca(OH)2 + CO2 ⟶ CaCO3 ↓ + H2O