2, 3, and 4 Marks Question
- Write any two features of modern periodic table.
-
Features of modern periodic table are:
- It is based on the atomic number.
- It separated the metals, metalloids and non-metals.
- Write two differences between IA group elements and VIIA group elements.
-
Two differences between IA group elements and VIIA group elements are shown
below:
IA Group VIIA Group They are metals. They are non-metals. Its reactivity increases from top to bottom. Its reactivity decreases from top to bottom. -
Which group of elements of the periodic table are kept in a given table?
What happens to the chemical reactivity of elements from top to bottom in
the given table? Why?
Group F Cl Br I - The elements of the periodic table which are kept in a given table belong to group VIIA or 17. The chemical reactivity of elements increases on moving from top to bottom in the given table because while moving from top to bottom, the atomic size increases with the increment in the number of shells and the force of attraction between the nucleus and valence shell decreases. Hence, the elements can lose the electron easily and become more reactive.
-
Study the given table and answer the given questions.
Name of elements Electronic configuration A 1s2, 2s22p6, 3s2 B 12, 2s22p6, 3s23p5 C 1s2, 2s22p6, 3s23p6, 4s1 -
- Write the name of elements indicated by A and B.
-
A = Magnesium
B = Chlorine - Keeping aluminum instead of A, which product would you expect? Write molecular formula of it.
- Keeping aluminum instead of A, Aluminum chloride is formed. Its molecular formula is AlCl3.
- Write valency of element C and chemical nature of it.
- Valency of element C is 1. It is alkali metal.
- Write the balanced chemical equation when 'C' reacts with 'B'.
-
K + Cl2 ⟶ KCl
2K + Cl2 ⟶ 2KCl
-
Study the electronic configuration of elements A and B given below and
answer the following questions.
A = 1s2, 2s22p6, 3s23p6, 4s1
B = 1s2, 2s22p4 -
- In which group of periodic tables do the elements A and B lie?
-
A = IA
B = VIA - Write the chemical reaction between A and B.
-
Na + O2 ⟶ Na2O
4Na + O2 ⟶ 2Na2O
- Write two characteristics of 'S' block elements.
-
Two characteristics of 'S' block elements are:
- They are metals.
- They are active.
-
Study the electronic configuration given below and answer the following:
A = 1s22S22P63S2
B = 1s22S22P63S23P5 -
- Write the name of elements A and B.
-
A = Magnesium
B = Chlorine - Write the balanced chemical equation of chemical reaction occurs between these two elements.
- Mg + Cl2 ⟶ MgCl2
- Chlorine is more reactive than nitrogen, why?
- Chlorine (Cl = 2,8,7) is more reactive than nitrogen (N = 2,5) because nitrogen needs 3 electrons to fulfil its valence shell but chlorine needs only one electron to fulfil its valence shell. One electron can be easily attracted with less force than 3 electrons, so chlorine is more reactive.
-
Study the part of periodic table given below and answer the questions:
IA IIA IVA VA VIA VIIA O Li Be C N O F Ne Na Mg Sl P S Cl Ar -
- Identify the group of very active metal and very active non-metal.
- The group of very active metal is Group IA and very active non-metal is Group VIIA.
- Write the electronic configuration of element 'S' in sub-shell.
- S = 1s22s22p63s23p4
- Why Na is more active than element Li?
- Na is more active than element Li because Na has bigger atomic size.
- Sketch the atomic structure of Cl.
-
The atomic structure of Cl is shown below:
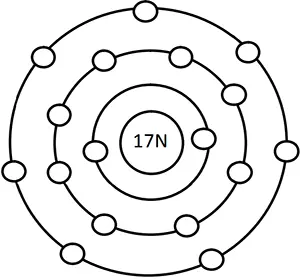
- What happens to the reactivity of metals from left to right in 3rd period of modern periodic table? Why?
- The reactivity of metals decreases because the atomic size decreases as we move from left to right in 3rd period of modern periodic table since the increase in the number of electrons adds to the same shell. It increases the power of nuclear attraction and decreases the tendency of donating electrons.
-
Study the given table and answer the following questions:
Elements Electronic configuration A 1s22s22p5 B 1s22s22p63s2 C 1s22s2sp63s23p5 -
- Write valency and block of element B.
- The valency of element B(Magnesium) is 2 and it lies in s-block.
- Why A is more reactive than C?
- A (Fluorine) is more reactive than C (Chlorine) because A has a small atomic size and the force of attraction of nucleus in the electron of the outermost shell is more. As a result, A gains an electron easily than C.
- Write balanced equation of chemical reaction between element B and C.
-
Balanced equation of chemical reaction between element B(Magnesium) and
C(Chlorine) is shown below:
Mg + Cl2 ⟶ MgCl2
- Atomic size of inert gases does not affect inertness, why?
- The inert gases do not affect inertness due to their electron configuration. Each of them has a full outer shell of electrons, which makes them highly unlikely to gain, lose, or share electrons which makes them unable to take part in the chemical reactions.
- Answer the following questions if atomic number of potassium is 19.
-
- Write the electronic configuration of it on the basis of sub-shell.
- Potassium = 1s22s22p63s23p64s1
- Write its group and period in periodic table.
- Potassium is in period 4 and group IA of periodic table.
- Write a balanced chemical reaction of potassium and oxygen.
-
K + O2 ⟶ K2O
4K + O2 ⟶ 2K2O
-
Which group of modern periodic table is shown in the given table and what is
the name given for metals in this group? What happens to the chemical
reactivity while going top to bottom in this group? Why?
Be Mg Ca Sr -
The group of modern periodic table shown in the given table is IIA and name
given for the metals in this group is alkaline earth metals.
The reactivity of elements increases on moving from top to bottom in this group because while moving from top to bottom, the atomic size increases with the increment in the number of shells and the force of attraction between the nucleus and valence shell decreases. Hence, the elements can lose the electron easily and become more reactive.
- What difference in chemical reactivity of metals of second period occur while moving from left to right in modern periodic table? Sodium is called metal, Why?
- Chemical reactivity of metallic element decreases from left to right in 2nd period. Sodium is called metal because it loses one electron from its outermost shell.
- In which group of modern periodic table, elements having atomic number 9 and 17 belong. Which one is more reactive and why?
- Elements having atomic number 9 and 17 belong to VIIA group of modern periodic table. The element having 9 atomic number is more reactive because it has small atomic size and the force of attraction of nucleus in electron of outermost shell is more than element having atomic number 17.
- What is the relationship between size of atoms and reactivity in case of non-metals?
- The relationship between size of atoms and reactivity in case of non-metals is that if size of atoms increases the reactivity decreases.
- Why reactivity of group VIIIA elements are not affected by their atomic size?
- Outermost shell of VIIIA group element contain 8 electrons so reactivity is not affected by atomic size.
- Between magnesium and calcium, which element is more reactive? Give reason.
- Between magnesium and calcium, calcium is more reactive because calcium has more shells, it is easier for it to lose electrons than magnesium.
-
Elements of which group in the periodic table are shown in the adjacent
table? Which elements is the most reactive among them? Give reasons. Write
the electronic configuration of Na and K on the basis of sub shell.
Group Li Na K -
Elements of group IA is shown in the adjacent table. K is the most reactive
element among them because it has bigger atomic size than other. So, it has
weaker attraction of force between nucleus and valence electrons which makes
easier to donate electrons.
Na = 1s22s22p63s1
K = 1s22s22p63s23p64s1 -
Study the table given below and answer the following questions.
Elements Electronic configuration P 1s22s22p63s1 Q 1s22s22p63s23p64s1 R 1s22s22p6 -
- What is the valency of element R?
- The valency of element R is 0.
- Which is more active between the element P and Q, why?
- Both P(Sodium) and Q(Potassium) are metal and in metal reactivity is directly proportional to the atomic size. Here, Q is bigger than P so it can donate electron easily. Hence, Q is more active.
- How many maximum electrons do d-subshell of periodic table have? Why are the element of group IA of this table called alkali metals?
- A d-subshell of periodic table have maximum 10 electrons. The element of group IA of this table called alkali metals because they react with water.
- Why does the reactivity of elements increase on moving from top to bottom in group IA of modern periodic table?
- The reactivity of elements increases on moving from top to bottom in group IA of modern periodic table because while moving from top to bottom, the atomic size increases with the increment in the number of shells and the force of attraction between the nucleus and valence shell decreases. Hence, the elements can lose the electron easily and become more reactive.
-
Answer the following question on the basis of given table.
Elements Electronic configuration A 1s22s22p63s1 B 1s22s22p63s23p5 C 1s22s22p63s23p5 -
- Write the name of elements A, B and C.
-
A = Sodium
B = Chlorine
C = Argon - To which block does the element 'C' belong? Mention its one chemical nature.
- Element 'C' belongs to p-block. It does not take part in chemical reaction.
- Hydrogen is kept with metals even it is a non-metal, why?
- Hydrogen is kept with metals even it is a non-metal because it behaves like a metal in quite a lot of chemical reactions. Its ion also has a positive charge like other metals.
-
Answer the following questions on the basis of the given table.
Name of element Electronic configuration X 1s22s22p63s1 Y 1s22s22p63s23p5 Z 1s22s22p63s23p6 -
- Write the name of elements indicated by X and Y.
-
X = Sodium
Y = Chlorine - Write the blocks of the elements X and Z.
-
X = s-block
Z = p-block - Write the valency and chemical nature of element Z.
- The valency is 0 and nature of element is inert of element Z(Argon).
- Distinguish between the modern periodic table and Mendeleev’s periodic table in two points. Elements of group VIIA are called noble gas, why?
-
Modern periodic table and Mendeleev’s periodic table are distinguished below
in table:
Elements of group VIIIA are called noble gas because they do not take part in chemical reaction.Modern periodic table Mendeleev's periodic table Elements are arranged in the increasing order of their atomic numbers. Elements are arranged in the increasing order of their atomic masses. There are a total number of 18 groups and 7 periods. There are a total number of 8 groups and 6 periods. - What is the change in chemical reactivity of very active non-metals with their atomic size increases?
- If the atomic size of very active non-metal increases then the chemical reactivity decreases because if atomic size increases the force of the attraction between the nucleus and valence shell decreases and the tendency of gaining electron also decreases.
- Elements of group VA, VIA and VIIA are less reactive as we go down in the group of the modern periodic table.
- Elements of group VA, VIA and VIIA are less reactive as we go down in the group of the modern periodic table because they contain non-metals and the tendency to gain the electrons decreases as we go down due to the increment in their atomic size and weak force of attraction between the nucleus and valence shell.
- Write any two difference between s and p-block element.
-
Difference between s and p-block element is shown below in table:
s-block element p-block element The elements whose last electron enters into s-orbital are called s-block element. The elements whose last electron enters into p-orbital are called p-block element. They have 1 or 2 electron in outermost subshell.
for example: hydrogen(H), helium(He) etc.They have 6 electron in outermost subshell.
for example: Boron(B), Neon(Ne) etc. - In which periodic table are the elements arranged on the basis of increasing atomic number? Why are group VIIA elements called halogens?
- In the modern periodic table, the elements are arranged on the basis of increasing atomic numbers. The group VIIA elements are called halogens because they react with metals to form compounds called salts.