2 Marks Question
- Though hydrochloric acid is formed inside our body, it is not called organic acid. Why?
- Organic acids contain carbon atoms in their molecules, but hydrochloric acid does not contain in its molecule. So, it is not considered an organic acid though it is formed in our body.
- Why is acid base reaction called neutralization reaction?
- Neutral substances are formed after the chemical reaction between acid and base. So acid-base reaction is called a neutralization reaction.
- Write down any two properties of acids.
-
Properties of acids are:
- Most of the acids are sour in taste.
- Strong acids are corrosive in nature.
Info: Acid change the color of litmus from blue to red, it react with base to form salt and water. - Write any two differences between neutral salt and acidic salt.
-
Any two differences between neutral salt and acidic salt is shown below in
table:
Neutral salt Acidic salt The salt formed by the complete displacement of hydrogen from acids is called neutral salt. The salt formed by the reaction between strong acid and weak base is called acidic salt. It does not have acidic properties. It has acidic properties. For example:
KOH + HCl ⟶ KCl + H2OFor example:
NaOH + H2SO4 ⟶ NaHSO4 + H2O - Why is H2SO4 considered as strong acid? What color does it give with phenolphthalein and methyl orange?
- H2SO4 is considered as strong acid because it gives more hydrogen ion (H+) when dissolved in an aqueous solution. It gives red color with methyl orange and become colorless with phenolphthalein.
- How is neutral salt formed? Give one example.
-
Neutral salt is formed by the complete displacement of hydrogen from acids.
For example,
KOH + HCl ⟶ KCl + H2O
KCl is neutral salt. - Why is acetic acid called weak acid?
- Acetic acid is called weak acid because it produces less hydrogen ion (H+ ion) in its aqueous solution.
- All alkalis are base but all bases are not alkalis. Justify this statement.
- All metallic oxides and hydroxides are called base but only water soluble metallic oxides are alkalis. Hence, all alkalis are base but all bases are not alkalis.
- Write an example of strong acid and strong base each. Give the balanced chemical equation of the reaction between them.
-
An example of strong acid is sulphuric acid (H2SO4),
hydrochloric acid (HCl) etc. and strong base is sodium hydroxide (NaOH),
potassium hydroxide (KOH) etc.
The balanced chemical equation of the reaction between them:
NaOH + HCl ⟶ NaCl + H2O - Explain why water can be considered as an acid as well as base?
- When water dissociates, it can form hydroxide and can also donate its hydrogen ion (H+ ion). Hence, it can be considered as an acid as well as a base.
- Why HCl is called as acid?
- HCl is called as acid because it produces hydrogen ion (H+ ion) in its aqueous solution.
- If two acids have pH values 2 and 5 respectively, which will be more acidic, why?
- Among the acids with pH 2 and 5, the acid with pH value 2 is more acidic because lower the pH higher will be the hydrogen (H+ ion) ion concentration in the solution.
- What is the pH value of substance formed by the chemical reaction between sodium hydroxide and hydrochloric acid?
-
Chemical reaction between sodium hydroxide and hydrochloric acid:
NaOH + HCl ⟶ NaCl + H2O
Here, sodium chloride and water are formed. So, the pH value of water and sodium chloride is 7. - Why is NaHSO4 called acidic salt?
-
NaHSO4 is called acidic salt because it is formed by the chemical
reaction between strong acid and weak base. That is;
H2SO4 + NaOH ⟶ NaHSO4 + H2O - What is pH value of neutral substance? Write with an example.
- pH value of neutral substance is 7. For example: water (H2O), sodium chloride (NaCl) etc.
- Write the name of neutral substance formed by the reaction of HCl with NaOH. Write down a name of alkali which is used to balance the pH of human stomach.
-
The name of neutral substance formed by the reaction of HCl with NaOH is
sodium chloride (NaCl).
NaOH + HCl ⟶ NaCl + H2O
A name of alkali which is used to balance the pH of human stomach is Magnesium hydroxide (Mg(OH)2). - NaHSO4 is acidic salt. Write with reason.
-
NaHSO4 is called acidic salt because it is formed by the chemical
reaction between strong acid and weak base. That is;
NaOH + H2SO4 ⟶ NaHSO4 + H2O -
Study the following chemical reactions and answer the questions given below:
NaOH + HNO3 ⟶ NaNO3 + H2O
Ca(OH)2 + 2HCl ⟶ CaCl2 + 2H2O -
-
Write down the name of an acid and a base/alkali from the above
reactions.
The name of acid is HNO3 (Nitric acid) or HCl (Hydrochloric acid) and base/alkali is NaOH (Sodium hydroxide) or Ca(OH)2 from above reaction. -
Why is it suggested to use soap to a person suffering from bee or ant
bite?
An ant or honey bee injects formic acid which reacts with soap causing neutralization. So, it is suggested to use soap to a person suffering from bee or ant bite. -
'All the bases are not alkalies.' Justify it.
All the bases are not soluble in water. So, all the bases are not alkalies.
-
Write down the name of an acid and a base/alkali from the above
reactions.
- A chemical substance gives hydrogen ion and chloride ion when dissolved in water. Similarly another chemical substance gives sodium ion and hydroxide ion. Write their molecular formula and also chemical reaction between them.
-
A chemical substance which gives hydrogen ion and chloride ion when
dissolved in water is HCl and another chemical substance which gives sodium
ion and hydroxide ion is NaOH.
Chemical reaction:
HCl + NaOH ⟶ NaCl + H2O - Write any two chemical properties of base with formula equations.
-
-
It reacts with acid to give salt and water.
HCl + NaOH ⟶ NaCl + H2O -
It reacts with carbon dioxide to give carbonate.
Ca(OH)2 + CO2 ⟶ CaCO3 + H2O
-
It reacts with acid to give salt and water.
- Though both sodium oxide and mercury oxide are metal oxides, sodium oxide is called alkali but mercury oxide not. Why? Write reason.
- Alkalis are water soluble bases and they give hydroxyl ions in aqueous solution. Though both sodium oxides and mercury oxide are metal oxides, sodium oxide dissolves in water and gives hydroxyl ions whereas Na2O is called alkali but no HgO.
-
In the given figure, red litmus paper is inserted in solution and color
remains unchanged then what may be contained in the vessel among acid, base and
salt solution. How can it be further tested to confirm it?
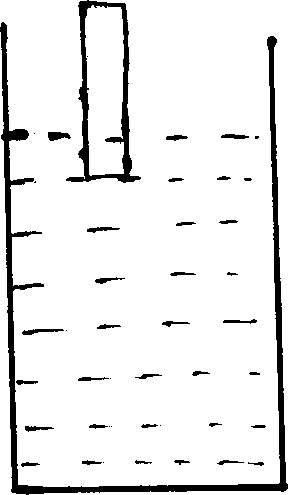
-
In the given figure, red litmus paper is inserted into the solution and color
remains unchanged then the solution may be acid or salt.
It can be further tested using blue litmus paper, if it is still blue then it is salt, if litmus paper changed into red then it is acid.