3 and 4 Marks Question
- How is coal formed in nature? Write in brief.
- Millions and millions of years ago, living beings (especially tall fern plants) were buried in the swampy land of earth. They were immediately covered by mud, stone etc. By extreme heat and pressure, carbon was extracted from them. It came from lignin, cellulose and hard parts. By slow chemical process, they were changed to coal. In this way, coal is formed in nature.
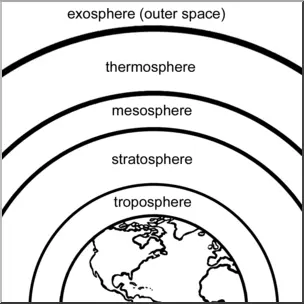
- Draw a labelled diagram of different layers of earth's atmosphere.
-
Diagram:
- Explain the formation and depletion of ozone layer with the chemical reaction.
-
Formation of ozone layer:
The ozone layer is present in the stratosphere. When the ultraviolet rays breakdown the oxygen molecules, nascent oxygen is formed which combines with a normal oxygen molecule to form ozone.
Reaction:
Depletion of ozone layer: By nitrogen oxide:
When nitrogen oxide are reached into the atmosphere it react with ozone and form oxygen with nitrogen dioxide. The nitrogen dioxide reacts with ozone layer and gradually destroys this layer.
Reaction:
NO + O3 ⟶ NO2 + O2
2NO2 + O3 ⟶ N2O3 + 2O2By chlorofluorocarbon (CFC):
When CFC are reached into the atmosphere they deplete the ozone layer. UV rays dissociate CFC and form nascent chlorine. The nascent chlorine reacts with ozone layer and gradually destroys this layer.
Reaction: